善纖達注射液(Saxenda®,サクセンダ)

善纖達Saxenda(Liraglutide)是一種用於成人體重管理的藥物。它是一種胰高血糖素樣肽-1(GLP-1)受體刺激劑,作用於大腦細胞中的GLP-1受體,刺激飽食中樞增加飽腹感並抑制食慾。雖然在歐洲和美國得到使用承認,但尚未在日本沒有厚生勞動省獲得使用核准,因此使用後出現不良反應,無法使用日本國內的藥品不良反應賠償制度。
體重減少率
在一個對3731名肥胖者分別注射Saxenda或安慰劑56週(約13個月)的研究中,92%注射Saxenda的病人平均減重8.4公斤(安慰劑平均為2.8公斤)。
| 體重減少率 | 5%以上 | 10%以上 | 15%以上 |
| 病人百分比 | 63.2% | 33.1% | 14.4% |
※如果在接受Saxenda治療4個月後,沒有觀察到體重比初始體重減輕3%以上,須考慮停止治療。
建議治療時間及停藥後體重變化
建議的治療時間為4-13個月,但也可以進行長期治療。 當停止Saxenda治療後,食慾會恢復正常,體重也容易恢復。 因此重要的是要把飲食和運動療法同時進行,養成一種健康減重的習慣。
Saxenda的使用和保存方法
- 請將未使用的Saxenda存放在冰箱裡,避免放置冷凍庫。 使用過的Saxenda應在室溫(2-30℃)下30天以內使用,避免暴露在直射日光下。 使用後,您可以繼續將Saxenda存放在冰箱裡保存。
- 每天早餐前同一時間在腹部、大腿或上臂施行一次皮下注射。請不要注射到肌肉或靜脈中。 請務必閱讀由診所所提供的注射說明書以及官方網站上的指導影片(點擊這裡觀看),並遵守使用說明。
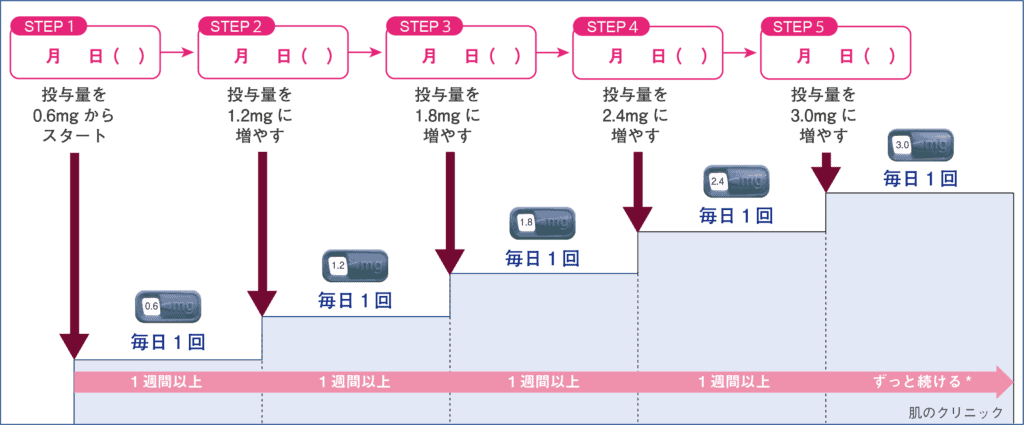
- 開始時一天注射0.6 mg,以適應其副作用。然後之後可視情況每星期增加0.6mg,最多不超過3.0 mg。如果減重效果顯著或噁心嘔吐等副作用持續存在,請不要增加劑量,繼續維持相同的劑量使用。
| 第1週 | 第2週 | 第3週 | 第4週 | 第5週以後 | |
| 1日注射劑量 | 0.6mg | 1.2mg | 1.8mg | 2.4mg | 3.0mg |
注射方式
注射Saxenda的方法請參考以下視頻。
如果您忘記Saxenda注射時
- 如果在原本的預定注射的12小時以內,請儘快注射原本預定的劑量,並在原本預計的時間注射下一次劑量。
- 如果離於本注射的時間超過12小時,請略過這一次注射。 在下一個原本預定的注射時間,注射上一次的(錯過的)劑量。 請不要一次注射兩倍的劑量。
- 如果超過三天沒注射,請由0.6 mg最低劑量重新開始注射。因為重新開始注射時比之前更可能出現噁心或其他症狀。
Saxenda的副作用
| 副作用分類 | 10%以上 | 1~10% | 0.1~1% | 0.01~0.1% |
|---|---|---|---|---|
| 免疫系統 | 過敏性休克 | |||
| 内分泌系統 | 甲状腺腫 | |||
| 代謝、營養 | 低血糖 | 脫水、高血脂症 | ||
| 精神 | 失眠 | |||
| 神經系統 | 頭痛 | 暈眩、味覺失調 | ||
| 心血管系統 | 低血壓 | 心悸頻脈 | ||
| 腸胃道系統 | 噁心、嘔吐、腹瀉、便祕 | 口渴、消化不良、胃炎、胃食道逆流、上腹部痛、腸脹氣、打嗝、腹脹 | 胰臟炎、胃排空延遲 | |
| 肝、膽道系統 | 膽結石 | 膽管炎 | ||
| 皮膚 | 蕁麻疹 | |||
| 腎臟 | 急性腎衰竭、腎功能受損 | |||
| 一般副作用 | 注射部位發紅、出疹、疼痛、内出血、硬結形成等、無力、倦怠感 | 情緒不穩 | ||
| 血液系統 | 胰臟酵素指數升高 | 貧血、肝指數上升 |
- 腸胃道症狀:噁心、嘔吐、腹瀉和便秘是最常見的副作用。 大多數是輕度到中度的,而且是暫時性的。 通常症狀會隨著治療的持續而減少。
- 低血糖:在非第2型糖尿病的患者中沒有嚴重低血糖的報告;1.6%的患者有低血糖症狀的報告(安慰劑組ˊ則是1.1%)。 如果出現強烈的饑餓感、冒冷汗、顫抖、心悸或發燒,請立即服用10克糖或100毫升含糖的果汁,然後休息。請務必在確認沒有低血糖症狀的狀態下,才可以駕駛汽車、操作機器,或進行高空作業。
- 胰臟炎、膽結石和膽囊炎:如果出現嚴重腹痛、背痛、嘔吐或發燒等症狀,因為有可能致命的風險請立即就醫,膽囊炎有時須切除膽囊。 膽結石可能發生在吃完油膩食物或暴飲暴食的晚上產生強烈腹痛。 在由膽結石引起的急性胰臟炎中,疼痛常是突然開始,並在幾分鐘內變成劇痛。 抽血檢驗澱粉酶和脂肪酶的升高不能預測胰臟炎的出現,但在出現症狀時是一個重要的診斷指標。
- 脫水和腎功能障礙:與胃腸道副作用如腹瀉和嘔吐有關的脫水可能進一步導致腎衰竭和腎功能障礙。 在出現腹瀉或嘔吐時,請多喝水補充水分。
- 過敏性休克/血管性浮腫:因藥物引起的過度免疫反應(過敏反應)可能導致血壓下降、昏厥、面部、嘴唇和皮膚水腫、皮疹和因呼吸道水腫而導致的呼吸困難等症狀。 如果出現過敏性症狀,需立即在醫院接受注射或其他治療,因此請叫救護車尋求協助。
- 腸阻塞(發生頻率不詳):如果出現嚴重便秘、腹脹、持續腹痛或嘔吐等異常情況,請停止使用Saxenda並就醫尋求協助。
- 憂鬱和自殺企圖:雖然因果關係不明,但被觀察到在0.1%的安慰劑與0.3%的Saxenda使用者中,出現了焦慮、虛弱、注意力不集中、抑鬱、易怒、情緒變化和自殺念頭。 如果出現症狀,請停止用藥,並立刻諮詢您的醫師。
- 心臟傳導障礙:在臨床試驗報告中,出現0.3%房室傳導阻滯、右枝阻滯和左枝阻滯,但與Saxenda的因果關係不明。
- 甲狀腺疾病:在第2型糖尿病患者的試驗中有出現甲狀腺腫的報告。 如果出現甲狀腺(在脖子前面,喉嚨下面)腫脹或腫塊等症狀,請務必諮詢您的醫師。 在對實驗鼠進行的為期兩年的致癌性研究中,有被報告非致命性的甲狀腺C細胞腫瘤(甲狀腺髓樣癌)。 在使用Saxenda的臨床試驗中也有甲狀腺乳頭狀癌(0.2%)的報告。
- 乳癌:臨床試驗顯示,有0.2%的安慰劑與0.7%的Saxenda使用者出現乳癌的報告,但病例數較少,因果關係尚未得到證實。 在那之後的研究也發現Saxenda與乳腺癌風險之間沒有關聯。
- 大腸腫瘤:臨床試驗顯示,有0.4%的安慰劑與0.6%的Saxenda使用者出現良性大腸腫瘤,0.1%的安慰劑至0.2%的Saxenda使用者出現惡性大腸腫瘤(大腸癌)。但上述報告的病例數都很少,因此因果關係不明。
- 胰臟癌:有報告,但與Saxenda的因果關係不明。
與其他藥物以及酒精的交互作用
如果在使用Saxenda時出現持續的腹瀉或嘔吐,warfarin(抗凝固藥物)的吸收可能受到影響。併用胰島素和其他糖尿病藥物時則有嚴重低血糖出現的報告。Saxenda與Orlistat(Xenical)沒有相互作用,但與其他減重藥物及減肥中藥的安全性尚未確定。
Saxenda與酒精沒有直接的交互作用,但過量飲酒會抑制肝臟中肝醣的產生和刺激葡萄糖的新生,並可能引起低血糖,因此接受Saxenda治療時應避免過度飲酒。
旅行時注意事項
- 在大多數情況下,國內航班不需要診斷證明書,但如果需要的話,請與我們聯絡。日文診斷證明書為3300日元(含稅),英文診斷證明書為5500日元(含稅)。
- 即使有時差,也請盡量在與日本同樣的預定注射時間注射Saxenda。 如果原本在日本預定的注射時間正好是您在當地的就寢時間,請在就寢前或起床後注射,回到日本後再恢復原本預定的注射時間。
懷孕和生育
懷孕和哺乳期內不能使用Saxenda。由於缺乏安全數據,本院建議男性和女性在治療期間應採取避孕措施,並在計畫懷孕開始前一個月停止使用。
不能接受Saxenda治療的對象
- 年齡未滿18歲或超過75歲的人。
- 患有糖尿病、胰臟炎、膽結石、膽囊炎、嚴重腎臟或肝功能障礙的人
- 有腹部手術史或腸阻塞病史者。
- 有厭食症,BMI低於18.5,體脂率男性低於15%,女性低於25%的人。
- 因內分泌失調或因藥物副作用(如類固醇)導致肥胖的人
- 患有甲狀腺疾病或有多發性內分泌腫瘤第2型家族史的人
- 孕婦和哺乳的婦女
- 有憂鬱症或自殺企圖的人。
- 對添加劑(二水磷酸氫二鈉、丙二醇、苯酚)過敏者。
料金(税込)
| 料金(税込) | |
|---|---|
| Saxendaサクセンダ (18mg) | 1枝注射筆 21,780円 |
| 檢查(必要時) | 抽血検査 3,982円 尿液検査(初診時女性) 1,056円 |
| 其他 | 注射針頭(70個) 2,860円 消毒酒精綿(100片) 660円 針頭回收容器(包含廢棄處理費用) 440円 止吐藥(10天分) 660円 |
※ 開立Saxenda需經過醫師診察後才能處方。本院就診須另收取醫師診察費用。(初診 3,850円、複診 1,650円)
※ 為了確認有無藥物副作用產生,在初診時,開始至料後一個月,以及之後每三個月,皆須進行抽血檢查。每次診察前也都會與您確認體重變化。
※ 針頭回收容器的設計是一旦蓋上蓋子就無法再打開。請務必將用過的針頭放入指定的容器中,最後蓋上蓋子並將其攜帶至診所廢棄。如果是其他醫療院所所處方的針頭,請將廢棄的針頭交由處方的醫院處理。如果您希望委託本院處理廢棄針頭,請購買本院所提供的針頭回收容器,並將針頭放入回收器後帶至本院。如果將廢棄針頭放入塑膠袋或寶特瓶中,會有造成針扎感染的風險,因此請不要使用本院所提供以外的容器收集廢棄針頭。請注意,如果希望由本院代為處理其他醫療院所所處方的針頭,須酌收醫療廢棄物處理費。
※ 請注意,即使是因為藥物副作用出現而無法繼續使用藥物,本院仍無法接受處方後的退還藥品及退款。敬請見諒。
需要30天的Saxenda的數量
| 維持劑量 | 30天所須的注射筆數量 |
|---|---|
| 0.6mg | 1枝 |
| 1.2mg | 2枝 |
| 1.8mg | 3枝 |
| 2.4mg | 4枝 |
| 3.0mg | 5枝 |
※ 如果從0.6mg開始,每週從1.2mg → 1.8mg → 2.4mg → 最大3.0mg增加劑量,32天的Saxenda注射筆3支就足夠了。
