What is Androgenetic Alopecia (AGA)?
Androgenetic Alopecia (AGA) is a progressive form of hair loss caused by the influence of DHT (dihydrotestosterone), a type of male hormone, which causes hair to become thinner and shorter until it eventually stops growing. This condition affects approximately 30% of Japanese men, and early treatment yields the best results.
Male Hair Loss Treatment: Evidence-Based Effective Therapies
AGA Treatment Case Study
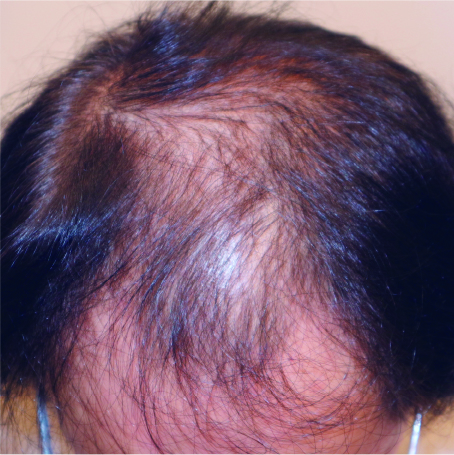
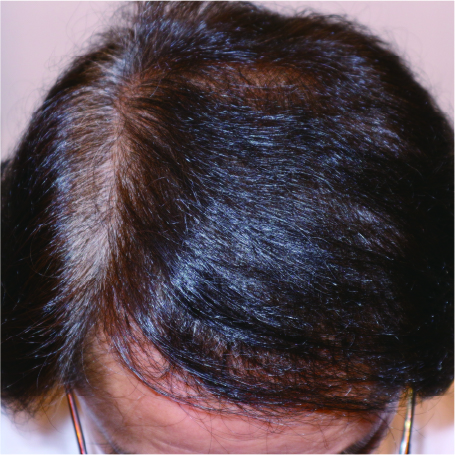
These before and after photos show the progress of a patient treated at our clinic with dutasteride, minoxidil tablets, and nizoral shampoo over 7 months. Treatment effects typically appear after about 6 months and peak after approximately one year. Continued medication is necessary to maintain results.
Standard Treatment | Finasteride + Topical Minoxidil
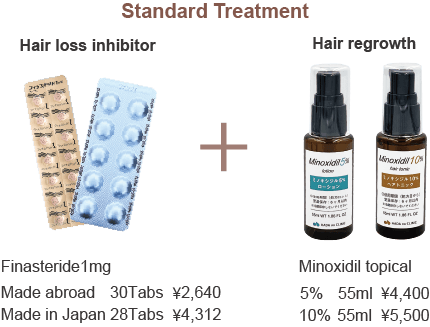
Our standard treatment combines oral finasteride (to inhibit hair loss) with topical minoxidil (to promote hair growth).
Finasteride suppresses the production of DHT, the primary cause of AGA, while topical minoxidil stimulates hair follicles to improve hair growth and thickness.
Recommended for:
- Patients with mild to moderate AGA symptoms
- Those concerned primarily with crown thinning
- Those who want to minimize side effect risks
Finasteride (Hair Loss Prevention Medication)
Characteristics
- FDA approved in 1997, approved in Japan in 2005
- 5α-reductase inhibitor that suppresses DHT production
- Take 1mg once daily
Finasteride Pricing (Revised May 13, 2023)
| Medication | Price (tax included) |
|---|---|
| Finasteride 1mg (Imported) | 30 tablets ¥2,640 |
| Finasteride 1mg (Japan) | 28 tablets ¥4,312 |
- Imported Finasteride 1mg is not an approved drug in Japan and is not covered by the Pharmaceutical Adverse Drug Reaction Relief System.
Effectiveness
Clinical trials report that after 1 year of use, 58% of patients showed improvement and 40% had halted progression.
Not Suitable For
- Individuals under 20 years of age
- Women (risk to fetal development)
- Men trying to conceive (discontinuation required one month before)
- Those with psychiatric disorders or sexual dysfunction
- Those with severe liver dysfunction
Side Effects
The incidence rate is approximately 6.5%, with sexual dysfunction (decreased libido 2.9%, erectile dysfunction 0.7%) being the primary side effects.
Topical Minoxidil (Hair Growth Stimulant)
Characteristics
- FDA approved in 1988, approved in Japan in 1999
- Topical hair growth agent that improves blood flow and stimulates hair follicles
- Our clinic offers 2%, 5%, and 10% concentrations (non-irritating formula)
Topical Minoxidil Pricing (Revised May 13, 2023)
| Medication | Price (tax included) |
|---|---|
| Minoxidil 2% | 1 bottle ¥3,300 |
| Minoxidil 5% | 1 bottle ¥4,400 |
| Minoxidil 10% | 1 bottle ¥5,500 |
- One bottle (55mL) of topical minoxidil lasts approximately one month.
- As these are compounded in-clinic, they are not approved drugs in Japan and are not covered by the Pharmaceutical Adverse Drug Reaction Relief System.
- Minoxidil is toxic to dogs and cats. Please take care not to let pets lick the solution or touch pets with hands that have been in contact with the solution.
Usage Instructions
Apply twice daily, using 5-6 pumps (approximately 1ml) per application to the affected areas, and massage gently with fingertips.
Effectiveness
Clinical trials with 5% topical minoxidil showed improvement in 40% of patients after 3 months, 80% after 6 months, and 90% after 12 months.
Not Suitable For
- Those with allergic tendencies
- Individuals under 20 or over 65 years of age
- Those with cardiovascular diseases
- Pregnant or nursing women
Side Effects
Scalp rash, itching, headache, and dizziness have been reported.
Standard Treatment Pricing
Example treatment cost when combining Finasteride 1mg and 5% Topical Minoxidil:
Initial Visit (2-month supply)
| Item | Price (tax included) |
|---|---|
| Initial Consultation | ¥3,850 |
| Medication | ・Imported Finasteride 60 tablets ¥5,280 ・Topical Minoxidil 5% 2 bottles ¥8,800 |
| Total (2 months) | ¥17,930 ¥8,965 per month |
Follow-up Visit (4-month supply)
| Item | Price (tax included) |
|---|---|
| Follow-up Consultation | ¥1,650 |
| Medication | ・Imported Finasteride 120 tablets ¥10,560 ・Topical Minoxidil 5% 4 bottles ¥17,600 |
| Total (4 months) | ¥29,810 ¥7,452 per month |
- Consultation fees apply for oral medication prescriptions. For topical medication prescriptions only, appointments are not necessary after the second visit.
- At the initial visit, we prescribe a 2-month supply to monitor side effects. If no problems arise at the follow-up, we can prescribe a 4-month supply, with consultations recommended every 3-6 months thereafter.
Advanced Treatment | Dutasteride + Oral Minoxidil

For those seeking more significant results, we offer advanced treatment with dutasteride and oral minoxidil.
Dutasteride has approximately 1.6 times the DHT suppression effect of finasteride, and oral minoxidil has a stronger hair growth effect than topical formulations, allowing for more powerful improvement.
Recommended for:
- Patients with moderate to severe AGA symptoms
- Those concerned with frontal hairline recession or M-shaped thinning
- Those with early-onset hair loss
- Those seeking more definitive hair growth results
Dutasteride (Advanced Hair Loss Prevention Medication)
Characteristics:
- Approved in Japan for AGA treatment in 2016
- Simultaneously inhibits Type I and Type II 5α-reductase (more powerful than finasteride)
- Take 0.5mg once daily
Dutasteride Pricing (Revised May 13, 2023)
| Medication | Price (tax included) |
|---|---|
| Dutasteride 0.5mg AV (Japan) | 30 tablets ¥3,960 |
- Dutasteride AV is prescribed off-label for AGA and is not covered by the Pharmaceutical Adverse Drug Reaction Relief System.
- Dutasteride AV is made in Japan, but due to limited supply, the form (capsule or tablet) or manufacturer may change. Products from different manufacturers may also be mixed.
Effectiveness
Compared to finasteride, dutasteride shows approximately 1.6 times improvement in hair count and 1.45 times improvement in hair thickness.
Not Suitable For
- Individuals under 20 years of age
- Women (risk to fetal development)
- Men trying to conceive (discontinuation required 6 months before)
- Those with psychiatric disorders or sexual dysfunction
- Those with severe liver dysfunction
Side Effects
Long-term administration studies report side effects in 16.7% of patients, primarily erectile dysfunction (10.8%), decreased libido (8.3%), and ejaculation disorders (4.2%).
Minoxidil Tablets (Oral Hair Growth Medication)
Characteristics
- Originally developed as antihypertensive medication, with confirmed hair growth effects
- Not yet approved for AGA, but safety and efficacy at low doses have been reported
- Requires regular examinations and consultations to manage side effects
Minoxidil Tablet Pricing (Revised May 13, 2023)
| Medication | Price (tax included) |
|---|---|
| Oral Minoxidil 2.5mg | 30 tablets ¥3,960 |
| Oral Minoxidil 5mg | 30 tablets ¥4,620 |
| Oral Minoxidil 10mg | 30 tablets ¥5,280 |
- Minoxidil tablets (oral medication) are not approved drugs in Japan and are not covered by the Pharmaceutical Adverse Drug Reaction Relief System.
Effectiveness
Offers the most powerful hair growth effect among existing AGA treatments.
Not Suitable For
- Individuals under 20 or over 65 years of age
- Those with cardiovascular or kidney diseases
- Pregnant, nursing, or trying-to-conceive women
- Men trying to conceive (discontinuation required one month before)
- Those unable to undergo regular examinations
Side Effects
Common side effects include hypertrichosis (excessive hair growth), edema, palpitations, and headaches. Though very rare, serious cardiovascular side effects such as heart failure and myocardial infarction can occur.
Advanced Treatment Pricing
Example treatment cost when combining Dutasteride 0.5mg, Minoxidil Tablets 2.5mg, and Nizoral Shampoo:
Initial Visit (2-month supply)
| Item | Price (tax included) |
|---|---|
| Initial Consultation | ¥3,850 |
| Medication | ・Dutasteride AV 60 tablets ¥7,920 • Oral Minoxidil 2.5mg 60 tablets ¥7,920 • Nizoral Shampoo 1 bottle ¥2,310 |
| Tests | ・Blood test ¥5,148 • Electrocardiogram ¥2,706 • Urine test ¥1,848 |
| Total (2 months) | ¥31,702 ¥15,851 per month |
Follow-up Visit (4-month supply)
| Item | Price (tax included) |
|---|---|
| Follow-up Consultation | ¥1,650 |
| Medication | ・Dutasteride AV 120 tablets ¥15,840 • Oral Minoxidil 2.5mg 120 tablets ¥15,840 • Nizoral Shampoo 2 bottles ¥4,620 |
| Tests | – ※1 |
| Total (4 months) | ¥37,950 ¥9,487 per month |
- Prescription of oral minoxidil requires tests (blood, electrocardiogram, urine) at the initial visit and once every 6 months.
- Consultation fees apply for oral medication prescriptions. For topical medication prescriptions only, appointments are not necessary after the second visit.
- At the initial visit, we prescribe a 2-month supply to monitor side effects. If no problems arise at the follow-up, we can prescribe a 4-month supply, with consultations recommended every 3-6 months thereafter.
Supplementary Treatment | Nizoral Shampoo & Ketoconazole Lotion
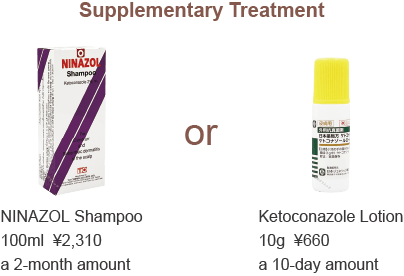
Nizoral Shampoo and Ketoconazole Lotion contain 2% ketoconazole, which has antifungal properties. They also have anti-androgenic effects, helping to suppress DHT externally to prevent hair loss. The lotion is recommended for those concerned about the smell or feel of the shampoo.
How to Use Nizoral Shampoo
Wet your hair, apply a small amount (size of a coin) to create lather on the scalp, and gently massage with your fingertips. Leave for 5 minutes before rinsing. For optimal results, use three times per week.
How to Use Ketoconazole Lotion
Apply 0.5mL (about half the size of a coin) to the scalp twice daily and massage gently. When used with minoxidil, apply ketoconazole first.
Scalp Micro Pigmentation (SMP)
Scalp Micro Pigmentation (SMP) is an effective complement to medication-based treatments. This procedure uses special pigments to create tiny dots on the scalp, producing the visual effect of higher hair density.
Recommended for:
- Those seeking immediate visual improvement alongside medication treatment
- Those with short hairstyles or shaved heads
- Those wanting to reduce the appearance of scalp showing through thinning hair
- Those wishing to conceal scalp scars
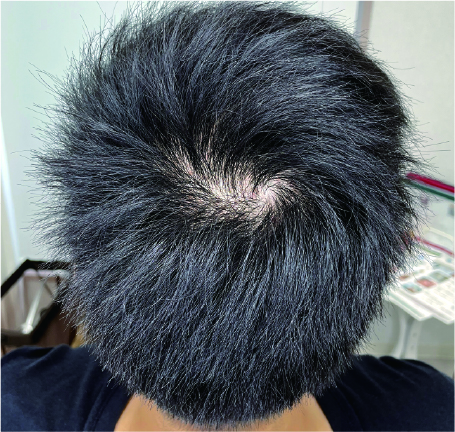
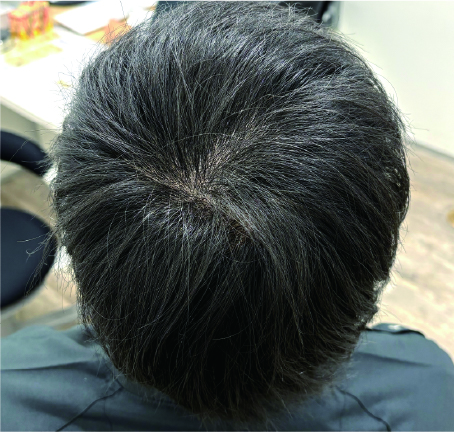
These photos show a patient at our clinic before SMP and one week after two SMP sessions on the crown area. The natural-looking results show an apparent increase in hair density and reduced scalp visibility.
Our Scalp Micro Pigmentation service prioritizes natural appearance and longevity. Using high-quality pigments and specialized techniques, we create the look of real hair follicles. Most patients see significant reduction in scalp visibility after an average of 3-4 sessions, helping restore confidence.For more detailed information, please visit our Scalp Micropigmentation page (available in Japanese only – please use translation tools).
References and Sites List
- B S Hugo Perez. “Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men” Med Hypotheses. 2004;62(1):112-5. doi: 10.1016/s0306-9877(03)00264-0. PMID: 14729013
- C Piérard-Franchimont et al., “Ketoconazole shampoo: effect of long-term use in androgenic alopecia” Dermatology. 1998;196(4):474-7. doi: 10.1159/000017954. PMID: 966913
- 前島 英樹ら, “壮年性脱毛患者に対するケトコナゾールローション外用の効果に関する検討” 西日本皮膚科 2007; 69: 182―185.
- Jaime R Fields, “Topical ketoconazole for the treatment of androgenetic alopecia: A systematic review” Dermatol Ther. 2020 Jan;33(1):e13202. doi: 10.1111/dth.13202. Epub 2020 Jan 2. PMID: 31858672
- Tater KC, Gwaltney-Brant S, Wismer T. Topical Minoxidil Exposures and Toxicoses in Dogs and Cats: 211 Cases (2001-2019). J Am Anim Hosp Assoc. 2021 Sep 1;57(5):225-231. doi: 10.5326/JAAHA-MS-7154. PMID: 34370845.
- プロペシア錠0.2mg/プロペシア錠1mg https://www.info.pmda.go.jp/go/pack/249900XF1021_3 最終アクセス 2023.02.10
- グラクソ・スミスクライン株式会社 医薬品インタビューフォーム ザガーロカプセル0.1mg ザガーロカプセル0.5mg 2021年11月改定
- Modha JD, Pathania YS. A comprehensive review of oral minoxidil in alopecia. J Cosmet Dermatol. 2022 Sep 6. doi: 10.1111/jocd.15324. Epub ahead of print. PMID: 36065675.
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J Am Acad Dermatol. 2021 Mar;84(3):737-746. doi: 10.1016/j.jaad.2020.06.1009. Epub 2020 Jul 2. PMID: 32622136.
- Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019 Aug;81(2):648-649. doi: 10.1016/j.jaad.2019.04.054. Epub 2019 May 2. PMID: 31054970.
- Hugo Perez BS. Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 2004;62(1):112-5. doi: 10.1016/s0306-9877(03)00264-0. PMID: 14729013.
- Pereira AFJR, Coelho TOA. Post-finasteride syndrome. An Bras Dermatol. 2020 May-Jun;95(3):271-277. doi: 10.1016/j.abd.2020.02.001. Epub 2020 Mar 25. PMID: 32317131; PMCID: PMC7253896.
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, Herlihy R, Fitch W, Labasky R, Auerbach S, Parra R, Rajfer J, Culbertson J, Lee M, Bach MA, Waldstreicher J; PLESS Study Group. Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003 Mar;61(3):579-84. doi: 10.1016/s0090-4295(02)02401-9. PMID: 12639651.




