Medical weight loss treatment at HADA NO CLINICSafe and Effective Medical Weight Loss Under Physician Supervision
At HADA NO CLINIC, we provide a safe and evidence-based medical weight loss program supervised by licensed physicians. Our approach includes regular medical check-ups and laboratory tests to ensure your health and safety throughout the treatment.
We use GLP-1 receptor agonists and other scientifically proven medications to support gradual and sustainable weight loss. Each plan is tailored to your individual needs, and combined with nutrition guidance and exercise support, we aim for long-term improvements in your body composition and metabolic health.
GLP-1 receptor agonists are particularly effective for appetite control and blood glucose regulation, making them a leading option in modern medical weight loss therapy.
Comprehensive Testing System for Safe Treatment
- Ongoing safety assessments and plan adjustments according to your progress
- Regular consultations and continuous health monitoring by medical professionals
- Comprehensive blood tests covering approximately 30 items, including liver and kidney function, and thyroid cancer markers
- Monitoring of blood glucose and HbA1c to detect and manage the risk of hypoglycemia
- Personalized treatment plans based on scientific data and clinical indicators
Weight Loss Medications Offered at Our Clinic
| Medication | Effect | Dosage | Features |
|---|---|---|---|
| Mounjaro | Weekly injection | Targets GIP/GLP-1 receptors to suppress appetite and increase energy expenditure | |
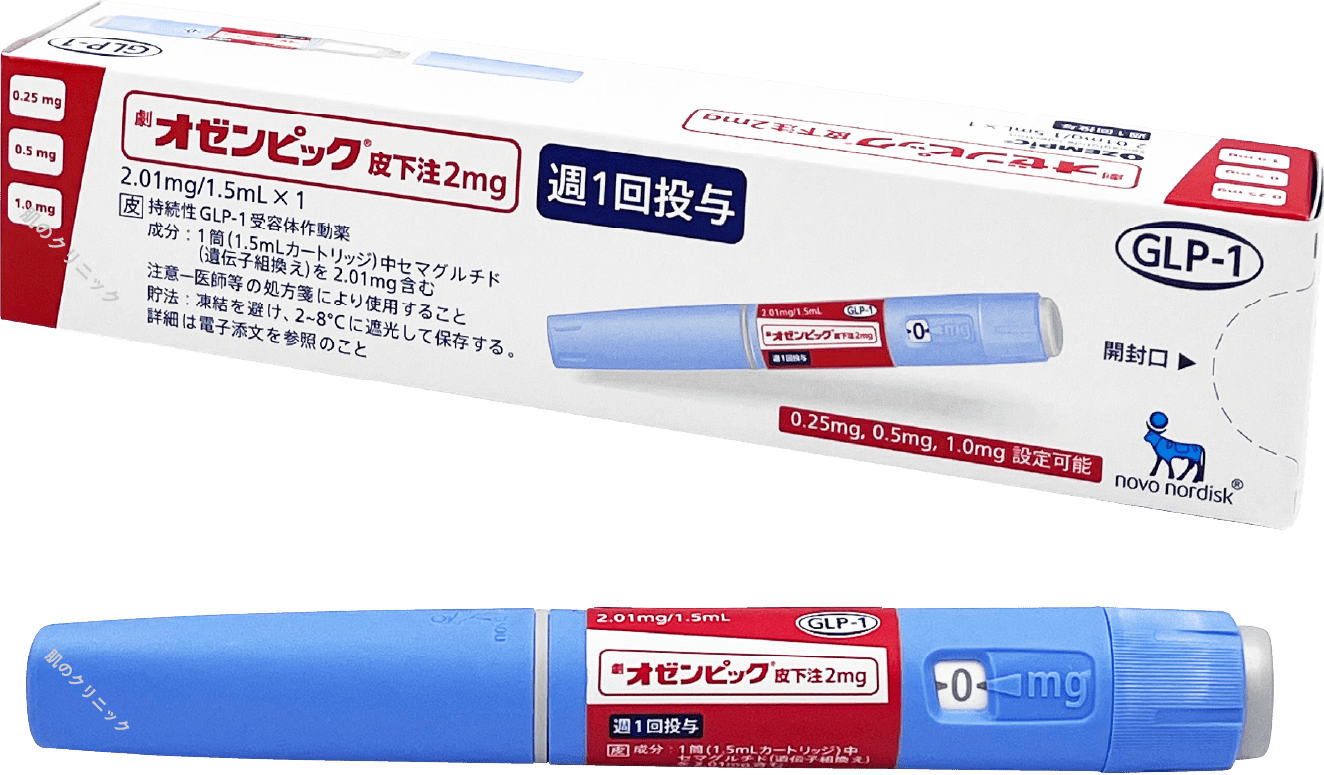
| Ozempic | Weekly injection | Targets GLP-1 receptors to suppress appetite and increase energy expenditure | |
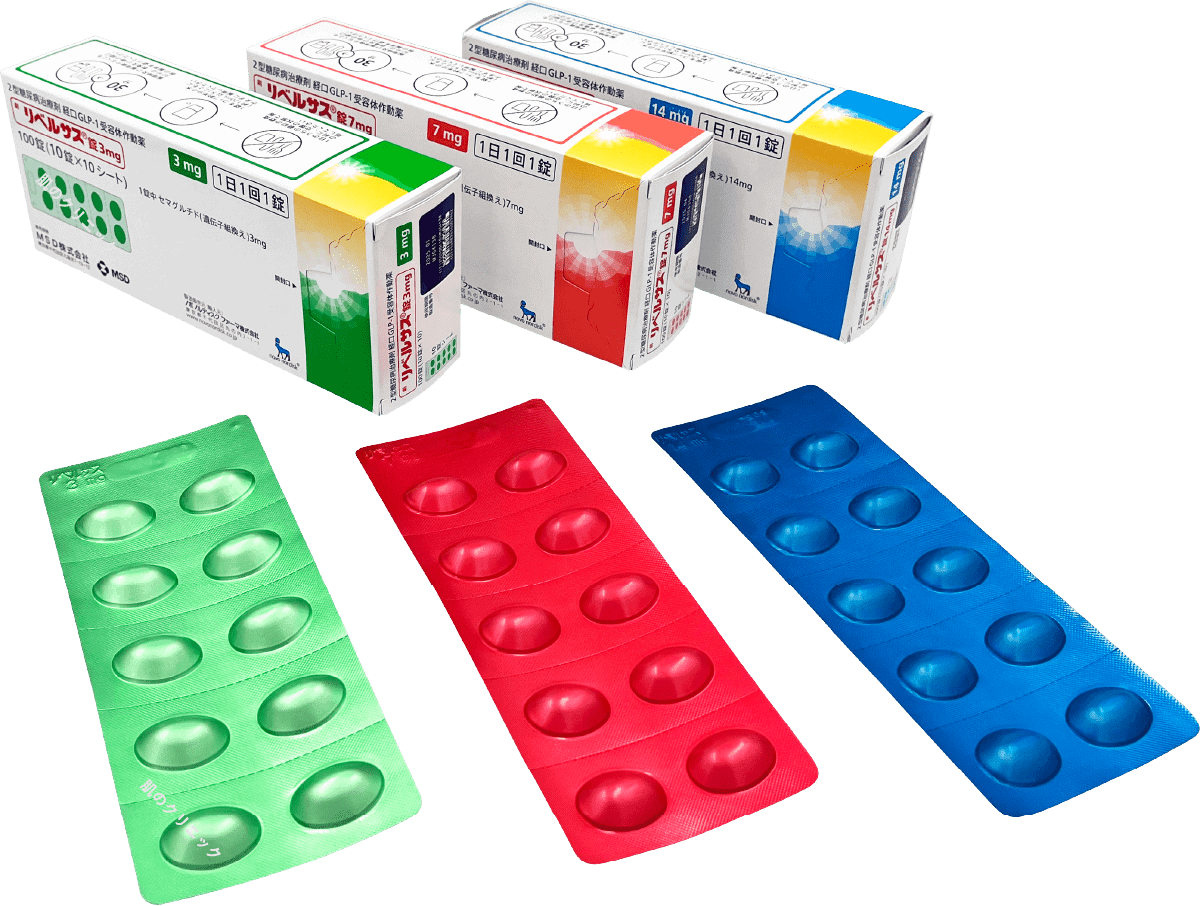
| Rybelsus | Oral, once daily | ||
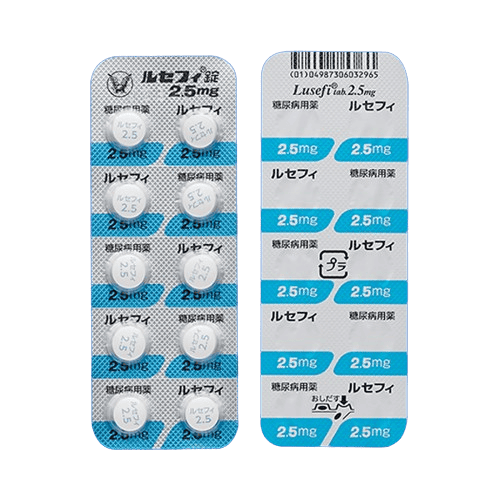
| Lusefi | Oral, once daily | Inhibits glucose reabsorption in the kidneys, promoting glucose excretion through urine | |
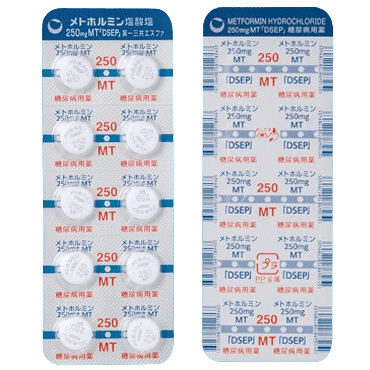
| Metformin | Oral, twice daily | Inhibits glucose production in the liver and reduces glucose absorption in the digestive tract Promotes GLP-1 secretion from the intestines to suppress appetite |
Mounjaro (Tirzepatide)
Mounjaro (Tirzepatide), also known internationally as Zepbound, is a once-weekly injection that helps suppress appetite and increase energy expenditure, aiding in weight loss. It works on both GLP-1 and GIP receptors, offering potentially greater weight loss effects compared to medications that target only the GLP-1 receptor.
While Mounjaro is approved as a treatment for diabetes, it has not yet been approved as a weight loss medication in Japan, meaning it is not covered by the Pharmaceutical Side Effect Relief System.
Mounjaro Prices (incl. tax)
| Drug | Price / pen (1 week) | Price / 4 pens (4 weeks) |
|---|---|---|
| Mounjaro 2.5mg | ¥5,500 | ¥22,000 |
| Mounjaro 5mg | ¥9,900 | ¥39,600 |
| Mounjaro 7.5mg | ¥14,300 | ¥57,200 |
| Mounjaro 10mg | ¥18,700 | ¥74,800 |
| Mounjaro 12.5mg | ¥23,100 | ¥92,400 |
| Mounjaro 15mg | ¥26,400 | ¥105,600 |
| Item | Price |
|---|---|
| Alcohol swabs (20 pieces) | ¥165 |
| Anti-nausea medication (10-day supply) | ¥660 |
- A consultation is required for a prescription (¥3,850 for the first visit / ¥1,650 for follow-up visits). Additionally, a blood test (¥3,982) is needed at the initial visit and every three months to monitor for side effects.
- After use, the needle retracts automatically into the pen for safety. Dispose of the pen in a sturdy container (like a milk carton), seal it with tape, and discard it as burnable trash. If you wish to dispose of items at our clinic, a medical waste fee of ¥440 will be charged.
Dosage and Administration
How to use Mounjaro
Storage:
Store in the refrigerator (2–8°C) and avoid freezing. It can also be stored at room temperature (below 30°C) for up to 3 weeks.
Injection Method:
Inject subcutaneously (into the fat) in the abdomen or thigh once a week, on the same day each week. It can be taken regardless of meals. Do not inject into muscles or veins. Follow the “Instructions for Use” provided during your consultation to use it correctly.
Dosage Schedule:
| Period | Dosage |
|---|---|
| Weeks 1–4 | 2.5 mg / week |
| Weeks 5–8 | 5 mg / week |
| Weeks 9–12 | 7.5mg / week |
| Weeks 13–16 | 10mg / week |
| Weeks 17–20 | 12.5mg / week |
| Week 21+ | 15mg / week |
Since nausea and discomfort may occur initially, start with 2.5 mg once a week and gradually increase the dose every 4 weeks according to the table, up to a maximum of 15 mg.
- If the effect is strong or side effects persist, maintain the current dosage without increasing it.
Who Should Not Receive Treatment
You may not be eligible for weight loss treatment if you meet any of the following criteria:
A. All Drugs (Mounjaro / Ozempic / Rybelsus / Lusefi / Metformin)
- Pregnant or breastfeeding women
- People with diabetes (consult at an insurance-covered facility)
- Those with severe kidney or liver issues
- Those with a history of abdominal surgery or intestinal obstruction (ileus)
- Those allergic to similar drugs
- Those at risk of hypoglycemia due to extreme exercise, excessive alcohol intake, pituitary/adrenal insufficiency
- Those who are underweight (BMI <18.5), have low body fat (<15% men, <20% women), or have eating disorders
In addition to the criteria in section A, you may not be eligible for treatment if:
Mounjaro / Ozempic / Rybelsus
- You meet any of the criteria in “A”
- You have severe gastrointestinal issues or a history of pancreatitis
- You have depression or suicidal tendencies
- You have thyroid issues or a family history of medullary thyroid cancer or MEN2
Side Effects
Mounjaro / Ozempic / Rybelsus
- 1. Gastrointestinal symptoms
-
Nausea, vomiting, diarrhea, constipation, abdominal discomfort, bloating. Usually temporary and improves with continued use.
- 2. Injection site reactions (excl. Rybelsus)
-
Redness, swelling, lumps. Usually temporary. Rotate injection sites.
- 3. Kidney issues
-
Dehydration from diarrhea/vomiting may cause kidney problems. Stay hydrated.
- 4. Increased heart rate
-
If sustained, stop the medication and consult a doctor.
- 5. Hypoglycemia
-
Symptoms include cold sweats, shaking, palpitations, hunger, headaches, drowsiness, seizures. Take 20g of sugar or 200ml juice and rest. Avoid driving or using machinery until symptoms pass.
- 6. Pancreatitis, gallstones, cholecystitis, cholangitis, jaundice
-
Rare but seek immediate medical attention for severe abdominal pain, back pain, vomiting, or fever. Rapid weight loss increases the risk of gallstones; keep weight loss under 5% per month.
- 7. Anaphylaxis, angioedema
-
Rare but seek emergency help for itching, hives, facial/throat swelling, difficulty breathing, dizziness.
- 8. Thyroid C-cell tumors (medullary thyroid cancer)
-
Cases in rats/mice, but human relevance is unclear. Report any neck swelling or lumps. Regular calcitonin blood tests will be conducted.
Pregnancy, Breastfeeding, and Fertility planning
These drugs are not for use during pregnancy or breastfeeding. Discontinue Mounjaro, Ozempic, or Rybelsus 2 months before trying to conceive, and Lusefi or Metformin 1 month before trying to conceive. No reported effects on male fertility.
Drug Interactions
Mounjaro / Ozempic / Rybelsus (Caution)
| Oral Contraceptives | May reduce the effectiveness of birth control pills. |
|---|---|
| Warfarin | May increase or decrease its effects. |
| Other Diabetes Medications | Higher risk of hypoglycemia. |
Alcohol Interaction
Moderate drinking is generally safe, but avoid heavy drinking as it can cause hypoglycemia.
【Recommended Limit】Up to 500ml of beer, 1 cup of sake, or 200ml of wine per day.
Interactions Between Diet Medications (Diabetes Drugs)
GLP-1 receptor agonists (Mounjaro, Ozempic, Rybelsus), SGLT2 inhibitors (Lusefi), and Metformin have a low risk of hypoglycemia alone but increase the risk when combined. Follow your doctor’s instructions and get regular check-ups and tests. If you are prescribed diet or diabetes drugs from other clinics, inform your doctor.
References & Website List
- Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022 Jan;70(1):5-13. doi: 10.1136/jim-2021-001952. Epub 2021 Oct 27. PMID: 34706925; PMCID: PMC8717485.
- Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, Helms HCC, Jeppesen JF, John LM, Pyke C, Nøhr J, Lu TT, Polex-Wolf J, Prevot V, Raun K, Simonsen L, Sun G, Szilvásy-Szabó A, Willenbrock H, Secher A, Knudsen LB, Hogendorf WFJ. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020 Mar 26;5(6):e133429. doi: 10.1172/jci.insight.133429. PMID: 32213703; PMCID: PMC7213778.
- US Food and Drug Administration . Wegovy prescribing information. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215256 最終アクセス2020.12.02.
- Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022 Jan 11;327(2):138-150. doi: 10.1001/jama.2021.23619. PMID: 35015037; PMCID: PMC8753508.
- novo nordisk “Wegovy semaglutide injection 2.4mg” https://www.wegovy.com/
- Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, Lingvay I, O’Neil PM, Rubino DM, Skovgaard D, Wallenstein SOR, Garvey WT; STEP 3 Investigators. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA. 2021 Apr 13;325(14):1403-1413. doi: 10.1001/jama.2021.1831. PMID: 33625476; PMCID: PMC7905697.
- Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, Lingvay I, Thomsen M, Wadden TA, Wharton S, Wilding JPH, Rubino D. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring). 2020 Jun;28(6):1050-1061. doi: 10.1002/oby.22794. PMID: 32441473; PMCID: PMC7318657.
- Buse JB, Bode BW, Mertens A, Cho YM, Christiansen E, Hertz CL, Nielsen MA, Pieber TR; PIONEER 7 investigators. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020 Dec;8(2):e001649. doi: 10.1136/bmjdrc-2020-001649. PMID: 33318068; PMCID: PMC7737050.
- Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D; STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. PMID: 33755728; PMCID: PMC7988425.
- Ghusn W, De la Rosa A, Sacoto D, Cifuentes L, Campos A, Feris F, Hurtado MD, Acosta A. Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity. JAMA Netw Open. 2022 Sep 1;5(9):e2231982. doi: 10.1001/jamanetworkopen.2022.31982. PMID: 36121652; PMCID: PMC9486455.
- Lingvay I, Hansen T, Macura S, Marre M, Nauck MA, de la Rosa R, Woo V, Yildirim E, Wilding J. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020 Oct;8(2):e001706. doi: 10.1136/bmjdrc-2020-001706. PMID: 33115821; PMCID: PMC7594204.
- Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konakli K, Lingvay I, McGowan BM, Oral TK, Rosenstock J, Wadden TA, Wharton S, Yokote K, Kushner RF; STEP 1 Study Group. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes Metab. 2022 Aug;24(8):1553-1564. doi: 10.1111/dom.14725. Epub 2022 May 19. PMID: 35441470; PMCID: PMC9542252.
- Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021 Jul 7;12:645563. doi: 10.3389/fendo.2021.645563. Erratum in: Front Endocrinol (Lausanne). 2021 Nov 10;12:786732. PMID: 34305810; PMCID: PMC8294388.
- ノボノルディスクファーマ 「ウゴービ® 肥満症治療剤 持続性GLP-1 受容体作動薬」https://pro.novonordisk.co.jp/products/wegovy.html 最終アクセス:2023.05.03
- ノボノルディスクファーマ 「ウゴービ® 添付文書」 chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://pro.novonordisk.co.jp/content/dam/hcpexperience/jp/ja/documents/products/wegovy/Wegovy_PI_latest.pdf.coredownload.inline.pdf 最終アクセス:2023.05.03
- O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018 Aug 25;392(10148):637-649. doi: 10.1016/S0140-6736(18)31773-2. Epub 2018 Aug 16. PMID: 30122305.
- Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 2017 Oct 17;318(15):1460-1470. doi: 10.1001/jama.2017.14752. PMID: 29049653; PMCID: PMC5817971.
- M.S. Capehorn, A.-M. Catarig, J.K. Furberg, A. Janez, H.C. Price, S. Tadayon, B. Vergès, M. Marre, Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10), Diabetes & Metabolism, Volume 46, Issue 2, 2020, Pages 100-109, ISSN 1262-3636, https://doi.org/10.1016/j.diabet.2019.101117. (https://www.sciencedirect.com/science/article/pii/S1262363619301326)
- ノボノルディスクファーマ株式会社 2型糖尿病治療剤 経口GLP-1受容体作動薬 セマグルチド(遺伝子組換え)リベルサス錠3mg リベルサス錠7mg リベルサス錠14mg 2021年9月改定(第2版)
- MSD Connect リベルサス 作用機序 https://www.msdconnect.jp/products/rybelsus/action_mechanism.xhtml 最終アクセス 2021/11/27.
- Ottney A. Glucagon-like peptide-1 receptor agonists for weight loss in adult patients without diabetes. Am J Health Syst Pharm. 2013 Dec 1;70(23):2097-103. doi: 10.2146/ajhp130081. PMID: 24249759.
- Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M; PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019 Sep;42(9):1724-1732. doi: 10.2337/dc19-0749.
- Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Baekdal T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021 Feb;23(2):581-588. doi: 10.1111/dom.14255. Epub 2020 Nov 27. PMID: 33184979; PMCID: PMC7839771.
- Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, Seino Y; PIONEER 9 investigators. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020 May;8(5):377-391. doi: 10.1016/S2213-8587(20)30075-9. PMID: 32333875.
- Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, Inagaki N; PIONEER 10 Investigators. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020 May;8(5):392-406. doi: 10.1016/S2213-8587(20)30074-7. PMID: 32333876.
- Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M; PIONEER 3 Investigators. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA. 2019 Apr 16;321(15):1466-1480. doi: 10.1001/jama.2019.2942. PMID: 30903796; PMCID: PMC6484814.
- Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya E; PIONEER 2 Investigators. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care. 2019 Dec;42(12):2272-2281. doi: 10.2337/dc19-0883. Epub 2019 Sep 17. PMID: 31530666.
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019 Aug 29;381(9):841-851. doi: 10.1056/NEJMoa1901118. Epub 2019 Jun 11. PMID: 31185157.
- 臨床応用可能な老化細胞除去薬の同定に成功―アルツハイマー病などの加齢関連疾患への治療応用の可能性 順天堂大学 日本医療研究開発機構 最終アクセス: 2024/10/15
- Katsuumi G, Shimizu I, Suda M, Yoshida Y, Furihata T, Joki Y, Hsiao CL, Jiaqi L, Fujiki S, Abe M, Sugimoto M, Soga T, Minamino T. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat Aging. 2024 Jul;4(7):926-938. doi: 10.1038/s43587-024-00642-y. Epub 2024 May 30. PMID: 38816549; PMCID: PMC11257941.
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. PMID: 23900241; PMCID: PMC3736576.
- Petrocelli JJ, McKenzie AI, de Hart NMMP, Reidy PT, Mahmassani ZS, Keeble AR, Kaput KL, Wahl MP, Rondina MT, Marcus RL, Welt CK, Holland WL, Funai K, Fry CS, Drummond MJ. Disuse-induced muscle fibrosis, cellular senescence, and senescence-associated secretory phenotype in older adults are alleviated during re-ambulation with metformin pre-treatment. Aging Cell. 2023 Nov;22(11):e13936. doi: 10.1111/acel.13936. Epub 2023 Jul 24. PMID: 37486024; PMCID: PMC10652302.
- Soukas AA, Hao H, Wu L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol Metab. 2019 Oct;30(10):745-755. doi: 10.1016/j.tem.2019.07.015. Epub 2019 Aug 9. PMID: 31405774; PMCID: PMC6779524.
- The TAME Trial Targeting the Biology of Aging. Ushering a New Era of Interventions. The official web resource of the TAME Trial, managed by the American Federation for Aging Research.